MAKING A DIFFERENCE
We put research, people and health systems to work for improved health and wellbeing
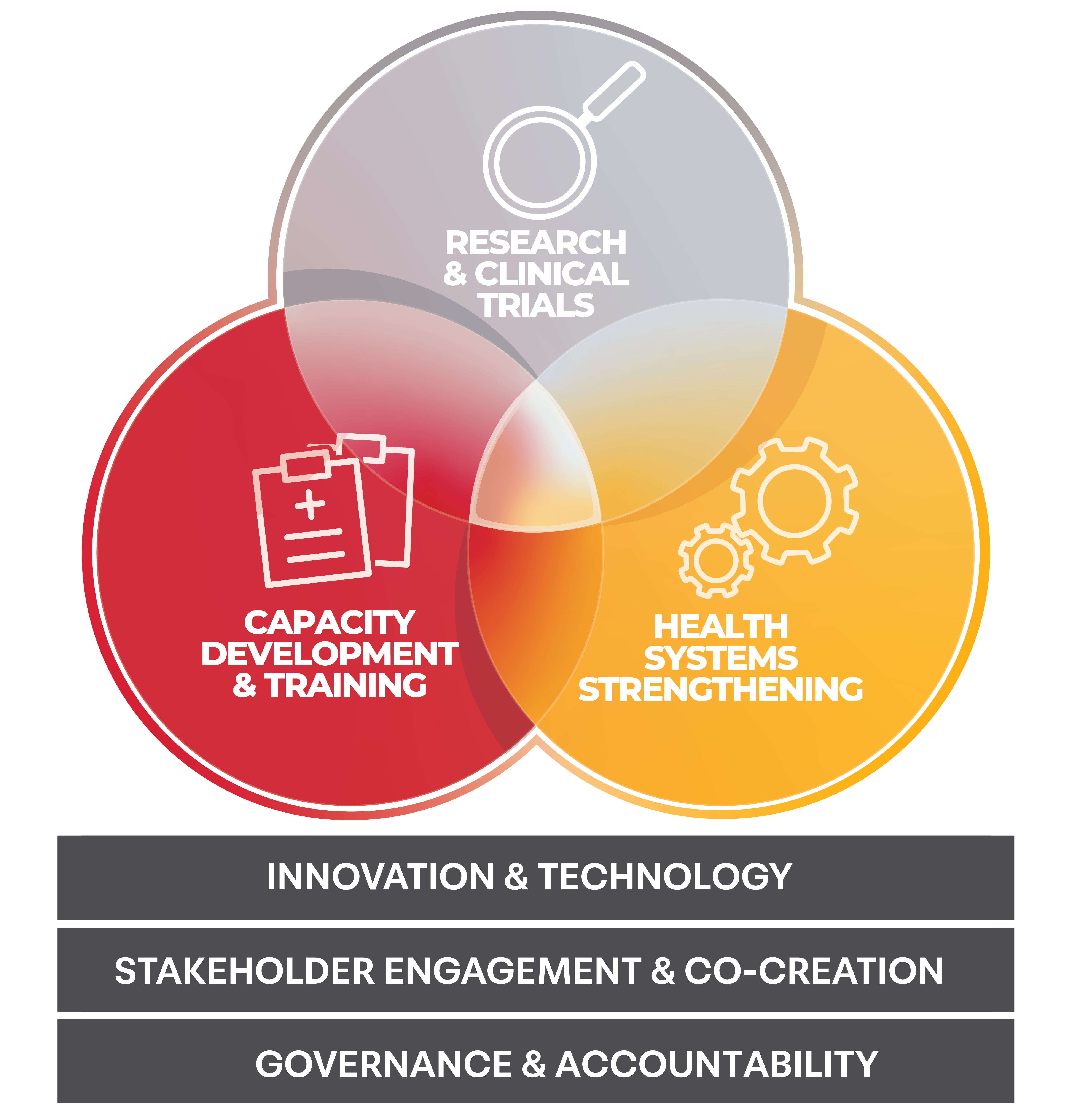
Combining genuine care for people with expertise and experience in three focus areas – Research & Clinical Trials, Capacity Development & Training and Health Systems Strengthening – we make a difference in the lives of people and communities affected by infectious disease.
We learn, develop and share ways to improve health care and roll up our sleeves to extend the impact of our work while envisioning a world free of suffering.