Focus on Digital Health Solutions and innovative Last Mile Delivery
THINK’s innovative health approaches and research has resulted in many initiatives with impact. Since 2014, THINK has implemented clinical trials that have brought forward the first three new TB drugs in 50 years. Our research on drug-resistant TB has informed the updated WHO guidelines to reduce treatment from 24 to 6 months. Other innovations include VDOT, CATT, Mobile digital X-rays, the impact of gender on treatment journeys and focusing on improving pediatric TB treatment.
Video Directly Observed Treatment (VDOT) for TB

Video Directly Observed Treatment (VDOT) for TB – Since 2014, THINK has been a strong advocate for the use of VDOT to support people receiving TB treatment. THINK has piloted the use of asynchronous VDOT in clinical trials in South Africa and interviewed patients and care-givers about the feasibly and acceptability in order to learn from and motivate for programmatic uptake in both TB and HIV programmes for differentiated treatment support.
Comprehensive Active TB Tracker (CATT)
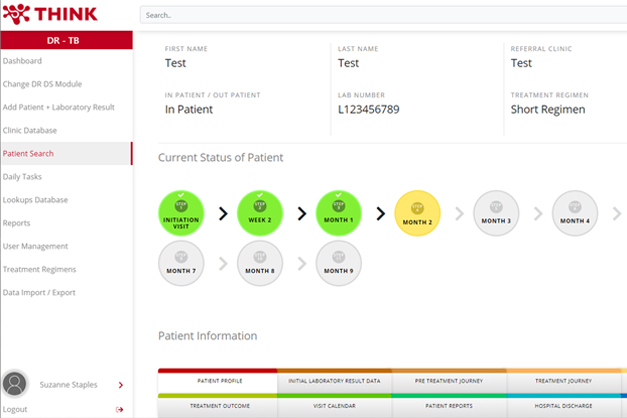
Comprehensive Active TB Tracker – CATT is currently piloted in programmatic setting under THINK. CATT is an innovative online application developed by THINK in 2020 to close the gaps in the health care cascade. The CATT system serves as a monitoring tool and checklist for healthcare providers to monitor contact screening, diagnosis, treatment, and adherence support, and provides prompts as reminders of upcoming activities and notifications of outstanding activities. CATT is currently piloted by THINK in a programmatic setting with funding from USAID.
THINK’s Mobile Clinics with Digital X-ray

THINK has acquired a fleet of mobile clinics that are fitted with Computer-Aided Detection (CAD) X-Ray and artificial intelligence (AI) to screen for TB in the lungs. These mobile clinics have proved to be instrumental in the detection of TB in remote and rural communities, thus finding missing TB cases and strengthening last mile delivery in South Africa. We are thankful to the USAID and the Japanese government for their support of this initiative.
Gender responsive interventions

Globally, we have a good understanding of how many women and men get sick with TB. However, there is lack of routine data on how the outcome of TB treatment differs by gender. This void led to a comprehensive social science study conducted by THINK to investigate TB treatment outcomes by gender and the different barriers men and women face in their treatment journey. The results have enabled THINK to design and pilot gender responsive interventions to improve TB treatment outcomes. This project is carried out with support from USAID in South Africa under the TB LON Programme.
Improved treatment and shorter duration of DR-TB treatment

Since 2014, THINK has implemented phase IIIII clinical trials to bring forward the first three new TB drugs in 50 years, including Bedaquiline, Pretonamid and Delamanid. Moreover, THINK has conducted the clinical trials that led the WHO to revise the international guidelines for TB treatment accordingly, first in 2019 and again in 2022.
These include the STREAM trial supported by USAID and Janssen Pharmaceuticals, which reduced duration of MDR-TB treatment from 2-years to 9 months and removed the need for injectable medication. More recently, with support from MSF, THINK completed the TB-PRACTECAL trial, which has reduced the duration of treatment even further to 6-months, using an all-oral regimen.
With a focus on children

THINK is proud to be one of the few organisations globally that focuses on the need to improve prevention, diagnosis and treatment for TB in children. THINK is currently implementing the TB CHAMP clinical trial to protect children from drug-resistant TB (University of Stellenbosch), THINK is also collaborating with the University of Stellenbosch, University of Wisconsin and TB Alliance to determine taste-preferences among children to improve palatability of TB medication. Furthermore, THINK is also conducting the TMC207 clinical trial with Janssen Pharmaceuticals to determine dose and safety of Bedaquiline in children ages 0-18 years. This study has been ongoing since 2014 and is THINK’s longest running clinical trial.


